Let’s discuss the question: how many unpaired electrons does magnesium have. We summarize all relevant answers in section Q&A of website Abettes-culinary.com in category: MMO. See more related questions in the comments below.
Does magnesium have any unpaired electrons?
1 Answer. In the ground state of magnesium, none of the valence electrons are unpaired.
How many unpaired electrons does manganese have?
Manganese atom has 5 electrons in 3d orbital and two electrons in 4s orbital.It has five unpaired electrons.
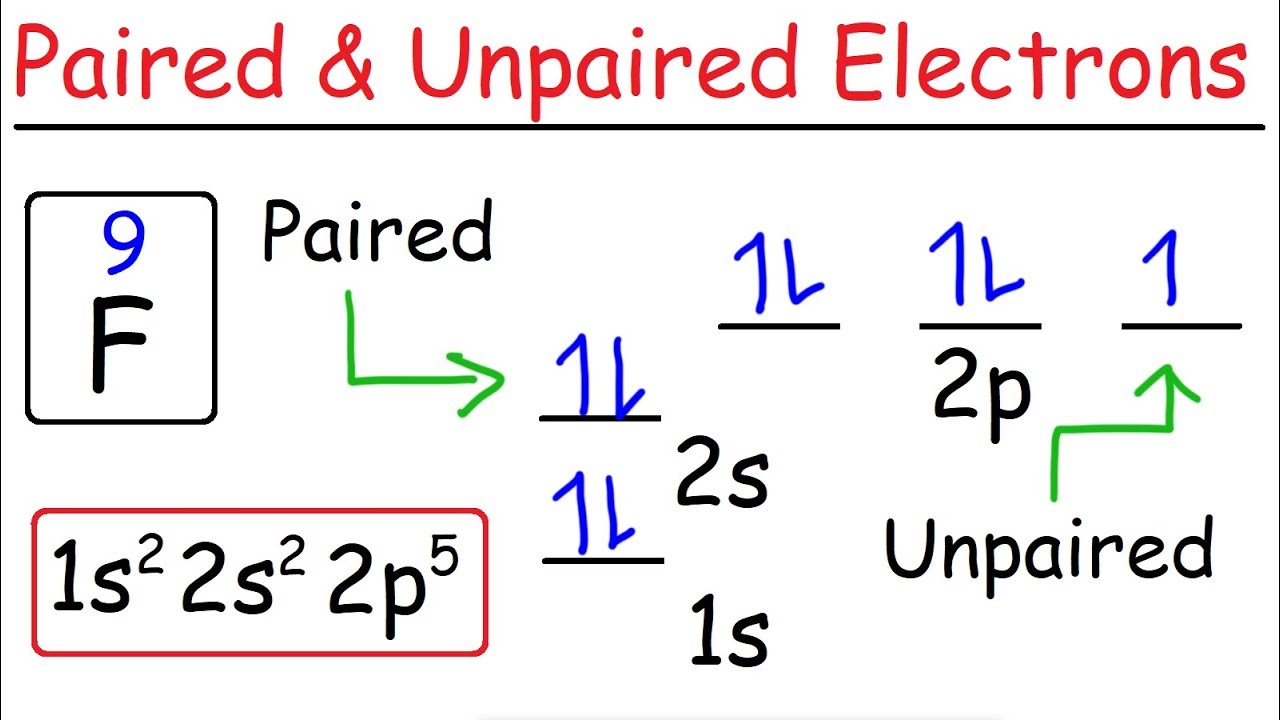
How To Determine The Number of Paired and Unpaired Electrons
[su_youtube url=”https://www.youtube.com/watch?v=xwWJf48yfNs”]
Images related to the topicHow To Determine The Number of Paired and Unpaired Electrons

How do you find the number of unpaired electrons?
Complete answer:
For finding the number of unpaired electrons, then first we have to find the atomic number of the element then write the configuration in the ground state, then according to the oxidation state subtract the number of electrons from the outer shell. So, there are 4 unpaired electrons.
How many unpaired electrons does Mg 2 have?
The electronic configuration of Mg2+ is 1s2 2s2 2p6. Since, the last shell is completely filled, there is no unpaired electron present.
What is the group number of magnesium?
| Group | 2 | 650°C, 1202°F, 923 K |
|---|---|---|
| Atomic number | 12 | 24.305 |
| State at 20°C | Solid | 24Mg |
| Electron configuration | Ne] 3s2 | 7439-95-4 |
| ChemSpider ID | 4575328 | ChemSpider is a free chemical structure database |
Is magnesium considered as a diamagnetic?
The magnesium metal is paramagnetic in spite of not having an unpaired electron.
What’s the electron configuration for manganese?
Is manganese a divalent ion?
Divalent manganese is the most stable oxidation state of manganese in acid and neutral aqueous solutions.
How many unpaired electrons does zinc have?
There are no unpaired electrons in Zinc. Zinc is unusually stable because of the lack of unpaired electrons.
What is meant by unpaired electrons?
In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Each atomic orbital of an atom (specified by the three quantum numbers n, l and m) has a capacity to contain two electrons (electron pair) with opposite spins.
What are unpaired electrons called?
● Atoms with unpaired ↑ electrons are called paramagnetic. ● Atoms with paired ↑↓ electrons are called diamagnetic.
How many unpaired electrons does bromine have?
Bromine has 1 unpaired electron in it’s orbital diagram which makes sense since bromide ions have a charge of -1 (they have gained 1 e– to complete their valence shell).
Paramagnetic vs Diamagnetic – Paired vs Unpaired Electrons – Electron Configuration
[su_youtube url=”https://www.youtube.com/watch?v=z7vvUaqu5As”]
Images related to the topicParamagnetic vs Diamagnetic – Paired vs Unpaired Electrons – Electron Configuration

How many valence electrons does magnesium have?
Magnesium has atomic number 12. Its electronic configuration is 2,8,2 . Thus, it has 2 valence electrons. It loses its two electrons to get stable or octet configuration.
What is the principal quantum number of magnesium?
From the periodic table, we see that the atomic number of magnesium is 12. Therefore, n = 3 (principal energy level for 3s) and l = 0 (for s orbital).
Is Mg2+ paramagnetic or diamagnetic?
Solution: Paramagnetic Species are those atoms, ions or radicals which have unpaired electrons. So, Na+ is diamagnetic because all its electrons are paired up. So, Mg+2 is also diamagnetic because all its electrons are paired up.
How many electron does magnesium have?
The most common and stable type of magnesium atom found in nature has 12 protons, 12 neutrons, and 12 electrons (which have a negative charge).
What is the composition of magnesium?
It reacts directly with many elements. Magnesium occurs in nature as a mixture of three isotopes: magnesium-24 (79.0 percent), magnesium-26 (11.0 percent), and magnesium-25 (10.0 percent).
What is magnesium in periodic table?
Chemical element, metallic, symbol Mg, situated in group IIa in the periodic table, atomic number: 12, atomic weight: 24,312. Magnesium is silvery white and very light. Its relative density is 1,74 and it’s density 1740 kg/m3 (0.063 lb/in3 or 108.6 lb/ft3).
Does magnesium have electrical conductivity?
…
Conductivity, Resistivity of Metals.
| Material | Resistivity p(Ω•m) at 20°C | Conductivity σ(S/m) at 20°C |
|---|---|---|
| Magnesium | 4.66×10–8 | 2.15×107 |
| Molybdenum | 5.225×10–8 | 1.914×107 |
| Iridium | 5.289×10–8 | 1.891×107 |
| Tungsten | 5.49×10–8 | 1.82×107 |
Is magnesium diamagnetic or paramagnetic Why?
Mg has paramagnetic property, in spite of not having single electron in its configuration and gold shows diamagnetic property in spite of having single electron in its S orbital.
Is MgO paramagnetic?
The paramagnetic defects in the magnesium-oxide (MgO) thin-film grown on a silicon (Si) substrate (MgO|Si) by a molecular beam epitaxy method are reported. These thin films have been used as an MgO tunnel barrier material for the spin-injection to Si from Fe.
How do you find the electron configuration?
To calculate an electron configuration, divide the periodic table into sections to represent the atomic orbitals, the regions where electrons are contained. Groups one and two are the s-block, three through 12 represent the d-block, 13 to 18 are the p-block and the two rows at the bottom are the f-block.
The number of unpaired electrons in magnesium atom is
[su_youtube url=”https://www.youtube.com/watch?v=UkTz-eZDFZE”]
Images related to the topicThe number of unpaired electrons in magnesium atom is

How many protons does manganese have?
What is the electron configuration chart?
| No. | Element | L |
|---|---|---|
| 8 | O | 2 4 |
| 9 | F | 2 5 |
| 10 | Ne | 2 6 |
| 11 | Na | 2 6 |
Related searches
- does mg have two unpaired electrons
- how many unpaired electrons does ga have
- how many unpaired electrons in magnesium
- unpaired electrons in manganese
- how many unpaired electrons does lu have
- how many unpaired electrons does p have
- how many unpaired electrons does br have
- what happens when magnesium loses 2 electrons
- how many unpaired electrons does germanium have
- how many electrons does magnesium lose
- how many unpaired electrons does nickel have
- how many unpaired electrons does y have
Information related to the topic how many unpaired electrons does magnesium have
Here are the search results of the thread how many unpaired electrons does magnesium have from Bing. You can read more if you want.
You have just come across an article on the topic how many unpaired electrons does magnesium have. If you found this article useful, please share it. Thank you very much.