Let’s discuss the question: how to set up ice table. We summarize all relevant answers in section Q&A of website Abettes-culinary.com in category: MMO. See more related questions in the comments below.
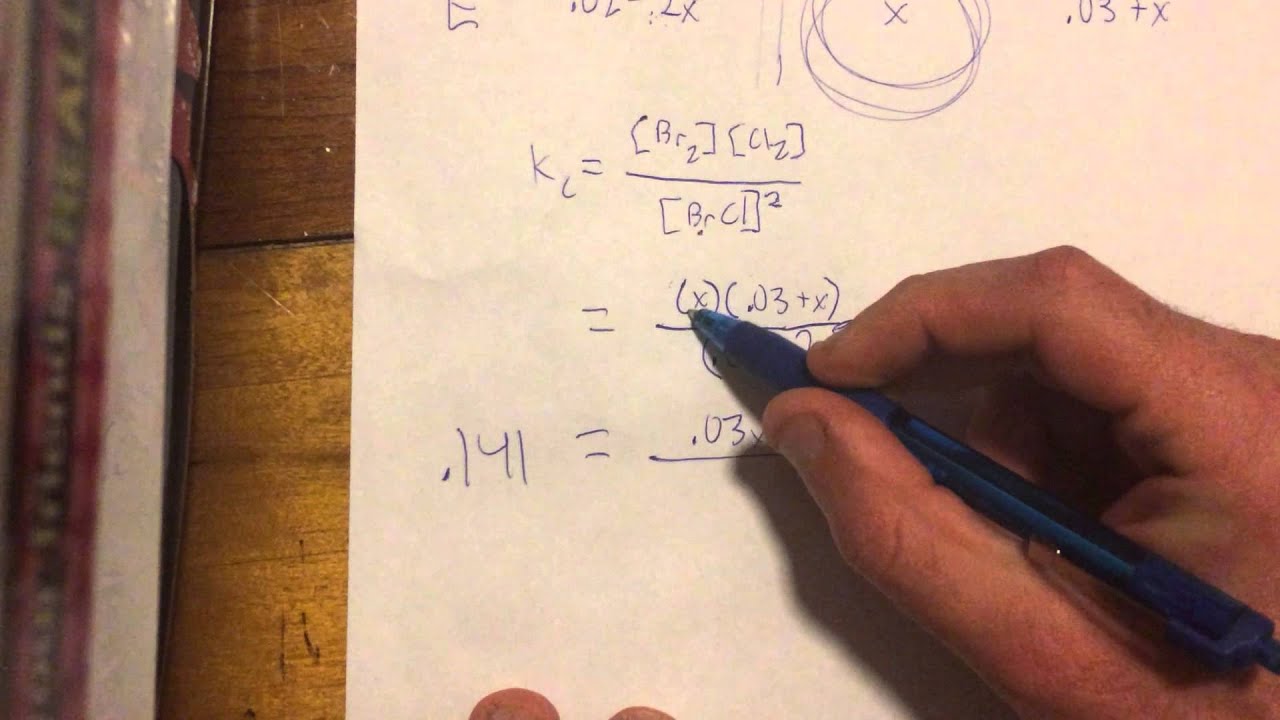
How does an ICE table work?
ICE tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are usually used to calculate the K, or equilibrium constant expression, of a reaction (in some instances, K may be given, and one or more of the concentrations in the table will be the unknown to be solved for …
What is the 5 rule in chemistry?
Lipinski’s rule states that, in general, an orally active drug has no more than one violation of the following criteria: No more than 5 hydrogen bond donors (the total number of nitrogen–hydrogen and oxygen–hydrogen bonds) No more than 10 hydrogen bond acceptors (all nitrogen or oxygen atoms)
ICE Tables made EASY!
[su_youtube url=”https://www.youtube.com/watch?v=YaOFe3J7jQE”]
Images related to the topicICE Tables made EASY!
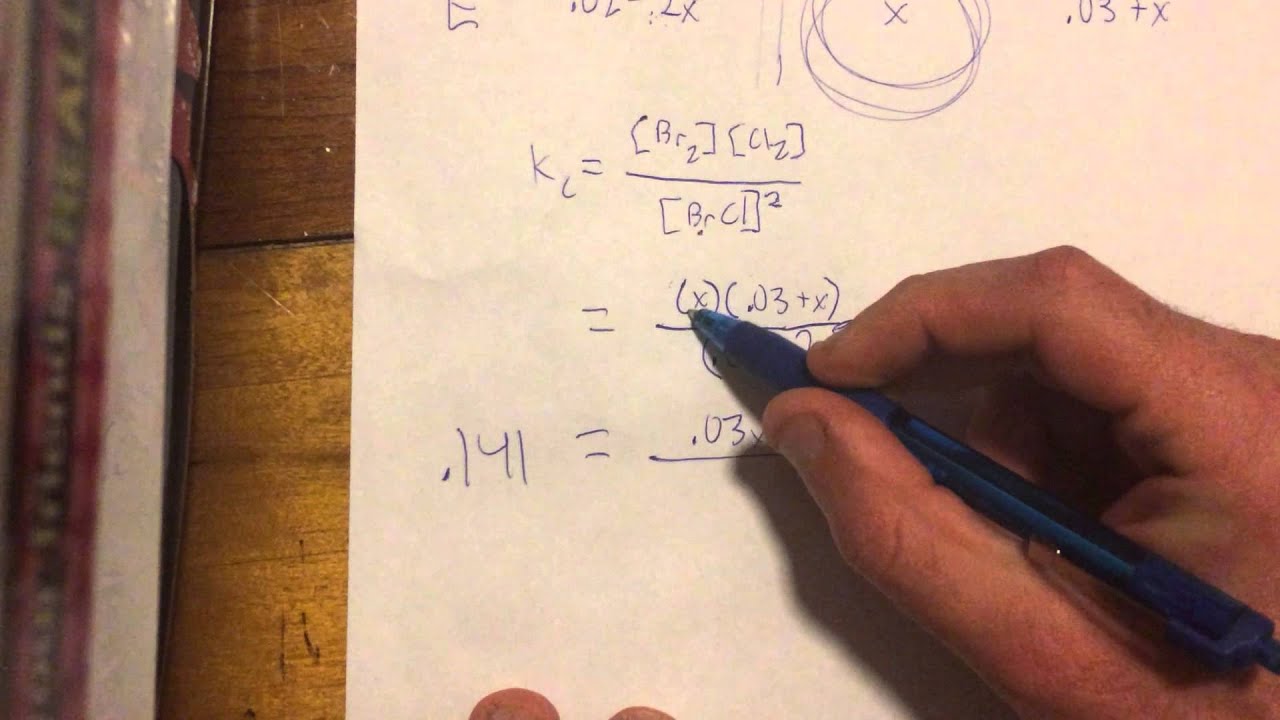
Do you include liquids in ice tables?
Note that no pure solids or liquids will appear in ICE tables because they do not appear equilibrium expressions. These kinds of problems will give the value of the equilibrium constant, Keq, in the problem, but NONE of the equilibrium concentrations are given.
What is KC formula?
K c = [ C ] c [ D ] d [ A ] a [ B ] b. If the equilibrium involves gaseous species, then the concentrations are replaced by partial pressures of the gaseous substances. The equilibrium constant in terms of partial pressures is: ⇒ K c = [ p C ] c [ p D ] d [ p A ] a [ p B ] b.
How do I calculate pH?
To calculate the pH of an aqueous solution you need to know the concentration of the hydronium ion in moles per liter (molarity). The pH is then calculated using the expression: pH = – log [H3O+].
What is the 100 rule in chemistry?
Although there is no explicit rule, for most practical purposes you can say that equilibrium constants within the range of roughly 0.01 to 100 indicate that a chemically significant amount of all components of the reaction system will be present in an equilibrium mixture and that the reaction will be incomplete or “ …
When can we use the 5% rule?
In calculating the pH of a weak acid or a weak base, use the approximation method first (the one where you drop the ‘minus x’). Then apply the 5% rule. If you exceed 5%, then you would need to carry out a calculation that does not drop the ‘minus x. ‘ This would result in quadratic equation, which would be solvable.
Introduction to I.C.E. Tables
[su_youtube url=”https://www.youtube.com/watch?v=VkKm0qELybE”]
Images related to the topicIntroduction to I.C.E. Tables

What is the Q in chemistry?
What is Q? The reaction quotient Q is a measure of the relative amounts of products and reactants present in a reaction at a given time.
What is the formula for partial pressure?
As has been mentioned in the lesson, partial pressure can be calculated as follows: P(gas 1) = x(gas 1) * P(Total); where x(gas 1) = no of moles(gas 1)/ no of moles(total).
What is Ka formula?
The Ka expression is Ka = [H3O+][C2H3O2-] / [HC2H3O2]. The problem provided us with a few bits of information: that the acetic acid concentration is 0.9 M, and its hydronium ion concentration is 4 * 10^-3 M. Since the equation is in equilibrium, the H3O+ concentration is equal to the C2H3O2- concentration.
How do you solve for equilibrium pressure?
The equilibrium partial pressure of each reactant will be the given initial partial pressure minus x. The equilibrium partial pressure of product will be x for reactions that have the first general form below or 2x for reactions that have the second form.
How do you find delta N?
Δn = (Total moles of gas on the products side) – (Total moles of gas on the reactants side). Hence \( \Delta = (d + c) – (a + b)\nonumber \] [The lower case numbers are the exponents]
What is difference between KP and KC?
The distinction between Kp and Kc is that Kp is the equilibrium constant with respect to atmospheric pressure, whereas Kc is the equilibrium constant with respect to the gaseous mixture’s molar concentration.
Chemical Equilibrium Constant K – Ice Tables – Kp and Kc
[su_youtube url=”https://www.youtube.com/watch?v=J4WJCYpTYj8″]
Images related to the topicChemical Equilibrium Constant K – Ice Tables – Kp and Kc

What is KP and KC?
Kp And Kc are the equilibrium constant of an ideal gaseous mixture. Kp is equilibrium constant used when equilibrium concentrations are expressed in atmospheric pressure and Kc is equilibrium constant used when equilibrium concentrations are expressed in molarity.
What is KP constant?
Kp is the equilibrium constant calculated from the partial pressures of a reaction equation. It is used to express the relationship between product pressures and reactant pressures. It is a unitless number, although it relates the pressures.
Related searches
- rice table calculator
- calculate the equilibrium constant kc
- ice table
- ice table chemistry worksheet
- how to set up ice table chemistry
- ice tables made easy
- ice table definition
- ice table chemistry worksheet pdf
- ICE table
- equilibrium constant
- how to create an ice table
- how to set up ice on samsung phone
- ice table calculator
- kc and kp
Information related to the topic how to set up ice table
Here are the search results of the thread how to set up ice table from Bing. You can read more if you want.
You have just come across an article on the topic how to set up ice table. If you found this article useful, please share it. Thank you very much.