Let’s discuss the question: how many molecules are there in 3.00 moles of nh3. We summarize all relevant answers in section Q&A of website Abettes-culinary.com in category: MMO. See more related questions in the comments below.
How many molecules are there in 3.4 moles of NH3?
Answer. Ammonia is NH3 The molar mass (reading from the periodic table) is 17.031g/mol. Therefore 3.4 grams of ammonia is equal to 0.1996359579590159 moles of ammonia. Multiplying this by 6.022 * 1023 we get 120220773882919374980000 molecules (or 1.2022 * 1023 molecules). …
How many molecules are in 3.00 moles Na?
1 mole of any element contains Avogadro’s number of atoms, that is, 6.022 x 10^23 atoms. Hence, 3 moles of sodium contains 1.8066 x 10^24 atoms and has a mass equal to 69 grams.
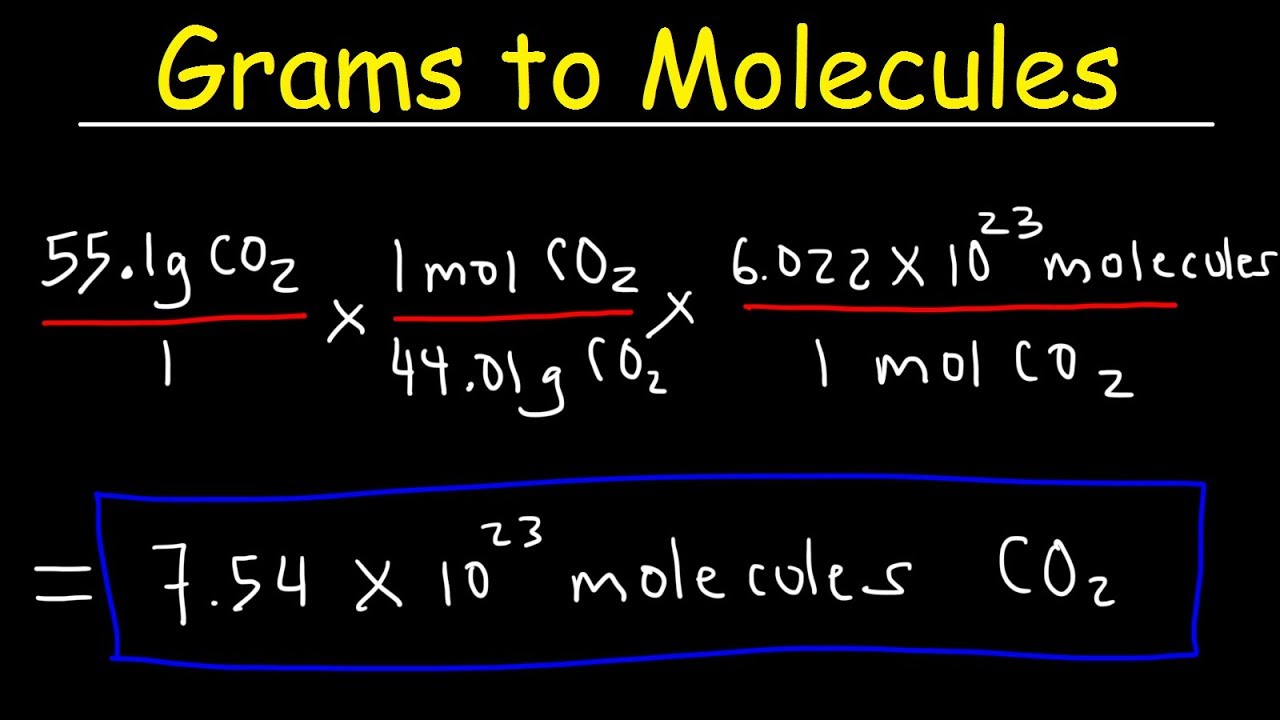
Grams to Molecules and Molecules to Grams Conversion
[su_youtube url=”https://www.youtube.com/watch?v=Ch16s4kYH1Q”]
Images related to the topicGrams to Molecules and Molecules to Grams Conversion

How many molecules is 3.00 moles?
1 Expert Answer
Do 3 moles x (6.022 x 10^23 molecules / 1 mol) to get about 1.8 x 10^24 molecules.
How many molecules are in a mole of NH3?
1 Expert Answer
molecules NH3 = 1.47 moles x 6.02×1023 molecules/mole = 8.9×1023 molecules of NH3 (to 2 sig. figs.)
How many grams is 3 moles?
molar mass of neon ( mass of one mole of neon) = 20 gram. Hence, mass of 3 mole of neon = 3 × 20 = 60 gram.
How many molecules are there in 25 mg of NH3?
There are 8.835 x 1023 molecules of NH3 N H 3 in 25 grams of NH3 N H 3 .
How many atoms are in 3.00 moles?
Explanation: 1 mole of O₂ contains 6.022 × 10²³ atoms. Thus, 3 moles O₂ would contain 3 × 6.022 × 10²³ atoms. Therefore, 3 moles of O₂ contain 18.066 × 10²³ atoms.
When the number of atoms in 3.0 moles of Na are found the answer is?
Hence, there are 18.066* 10²³ atoms in 3 moles of Sodium.
How many particles are in 2.2 moles sodium?
There are 1.20⋅1024 atoms of sodium in 2 moles of sodium.
How many molecules are there in 3.00 moles of water?
So, in 3 moles of the substance, there’ll be 3⋅6=18 moles of water molecules. Remember that a mole of molecules contains 6.02⋅1023 molecules. So here, there’ll be a total of 6.02⋅1023⋅18≈1.08⋅1025 water molecules.
How many molecules are there in 3 moles of CO2?
How many molecules are there in 3 mol CO2? There are 1.806 X 1024 molecules in 3 moles of CO2.
How many molecules of ammonia are in 10 moles of ammonia NH3?
1 Answer. 10 mol NH3 contains 6×1024molecules NH3 .
Avogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction
[su_youtube url=”https://www.youtube.com/watch?v=74-X94OP2XI”]
Images related to the topicAvogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction

How many molecules are in 2.5 moles of ammonia?
1.5×1024 molecules.
What is the mole of NH3?
17.03052 g/mol .
How can you represent 2 molecules of ammonia?
- This shows that ammonia exists as diatoms. …
- To achieve this, nitrogen shares three of its electrons with three hydrogen atoms. …
- This separate pair of electrons of one is shared with the lone pair of another molecule to form a stable compound.
How many molecules are in a mole?
The mole is represented by Avogadro’s number, which is 6.022 × 1023 atoms or molecules per mol.
What is the exact weight of 3 moles of any?
Mass of 3 moles of N =14×3=42 g.
What is the mass of 3 mole of nitrogen?
∴ 3 moles of N2 weighs =3×28g=84g.
How many molecules are in a 34 g sample of ammonia NH3?
therefore 34 g of NH₃ contains 12.044*10²³ molecules .
How many molecules are there in 4 moles of glucose?
How many molecules are there in 4.00 moles of glucose, Ch,o 4.00 moles 6.022X 102 molecules 2.41 * 102 molecules 00 x 6 022 EXP 233241E24 mole When the units are set up properly, the unit you are converting FROM will cancel out: You are left with the desired unit you are converting TO 3.
How many molecules of ammonia NH3 are produced?
By chemical reaction for the formation of ammonia, 3 moles of hydrogen produces 2 moles of ammonia. Thus, 0.33 moles of ammonia or 2 x 10^23 molecules (approximately) are produced.
How many atoms are in 3.00 moles of Sn?
1 Answer. There are 3×NA tin atoms in 3.0 mol of tin metal, where NA is Avogadro’s number, 6.022×1023 mol−1 .
How to Calculate the Number of Molecules in Moles of Carbon… : Chemistry and Physics Calculations
[su_youtube url=”https://www.youtube.com/watch?v=xDXOYGdFTEI”]
Images related to the topicHow to Calculate the Number of Molecules in Moles of Carbon… : Chemistry and Physics Calculations

How many atoms does NH3?
Ammonia, NH3, is a chemical compound composed of one nitrogen atom and three hydrogen atoms.
How many atoms are in 3 CO2 molecules?
How many atoms are in 3 moles of CO2? 1 molecule of CO2 = 1 C atom + 2 O atom. So, 3 moles of CO2 contains (3 x 2 x 6.02 x 10*23) O atoms = 3.61 x 10*24 O atoms.
Related searches
- how many molecules are in 30 8 moles of nh3
- how many molecules are present in 3 25 mol of c2h6o
- how many moles of nahco3 are present in a 2 00 g sample
- how many molecules are in 3 moles of nh3
- how many molecules are there in 3.4 moles of nh3
- how many molecules are there in 3.00 moles of nh3
- how many molecules are present in 3.25 mol of c2h6o?
- how many molecules are there in 3.00 moles of nh3 quizlet
- calculate the number of moles of aspirin c9h8o4 in 4 0 gram sample
- how many molecules are there in 3 00 moles of nh3 quizlet
- how many kilograms of lithium are in a 0 653 mole sample
- how many molecules are there in 3.2 moles of ammonia nh3
- how many molecules are in 30.8 moles of nh3
- how many moles are there in 3g of h2o
- how many moles of nahco3 are present in a 2.00 g sample?
- how many molecules are there in 3 2 moles of ammonia nh3
Information related to the topic how many molecules are there in 3.00 moles of nh3
Here are the search results of the thread how many molecules are there in 3.00 moles of nh3 from Bing. You can read more if you want.
You have just come across an article on the topic how many molecules are there in 3.00 moles of nh3. If you found this article useful, please share it. Thank you very much.