Let’s discuss the question: how many moles is 3.52 x10 24 molecules of water. We summarize all relevant answers in section Q&A of website Abettes-culinary.com in category: MMO. See more related questions in the comments below.

How many mol are 4.52 x10 24 molecules H2O?
4.65×1024⋅NO2⋅molecules6.022×1023⋅NO2⋅molecules⋅mol−1=7.72⋅mol …
How many moles is 1.5 x10 23 molecules of water H2O?
( 1/4 of a mole) x (6.02 x 1023 atoms/mole) = approximately 1.5 x 1023 atoms. If you have a compound like H2O, then: one mole of water contains 6.02 x 1023 MOLECULES of water.
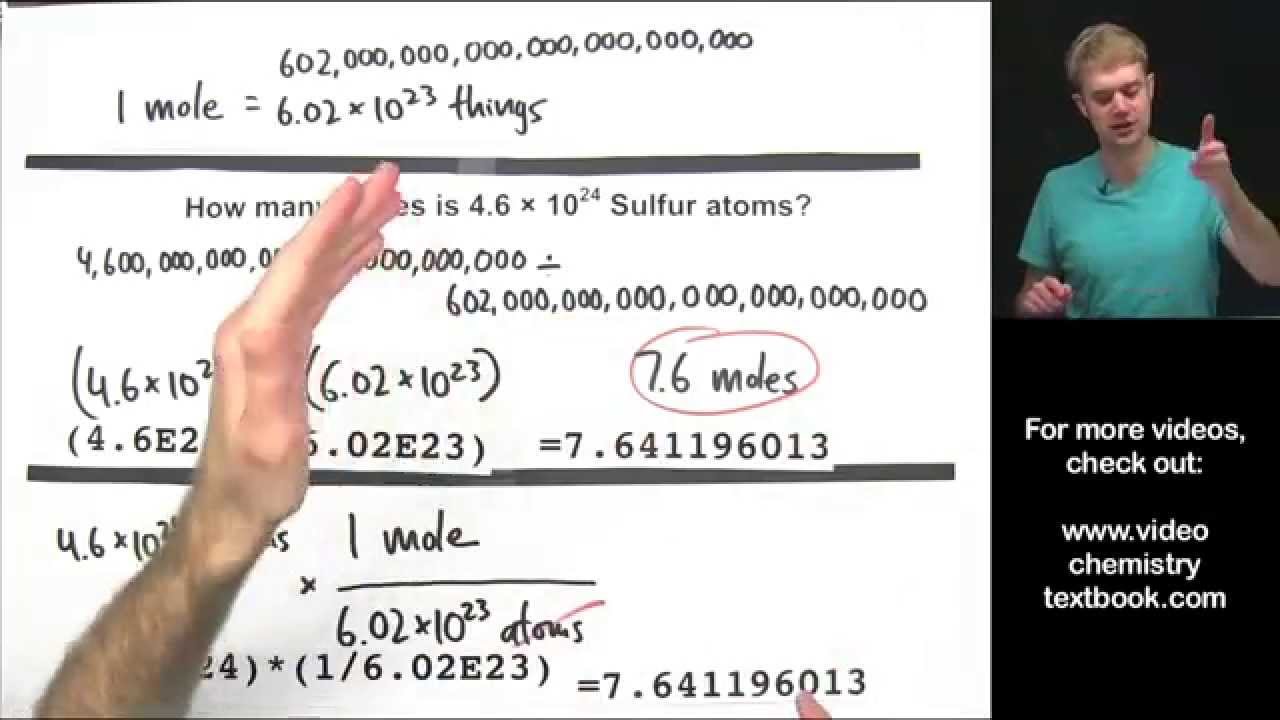
Avogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction
[su_youtube url=”https://www.youtube.com/watch?v=74-X94OP2XI”]
Images related to the topicAvogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction

How many moles are 6.02 x10 23 molecules of water H2O?
Answer 4: That’s 0.1 mole of water, because 6 x 1023 is the number of molecules or atoms in a mole.
How many moles are in the 1.204 x10 24 molecules?
1.204×1024 molecules = 2.00 moles.
How many moles of glucose does 1.2 x10 24 molecules represent?
How many moles of glucose does 1.2 x 1024 molecules represent? 1 mole of any substance has 6.02 x 1023 molecules. For getting thenumber of moles we can divide 1.2 x 1024/6.02 x 1023 and we receive 2 moles.
What is 1 mol H2O?
The average mass of one H2O molecule is 18.02 amu. The number of atoms is an exact number, the number of mole is an exact number; they do not affect the number of significant figures. The average mass of one mole of H2O is 18.02 grams.
How many moles of CaCl2 does 2.41 x10 24 formula units represent?
16) How many moles of CaCl2 does 2.41 x 1024 formula units represent? 1100.
How many water molecules are in 5.2 moles water?
Answer. Answer: There are therefore 6.02 × 1023 water molecules in a mole of water molecules.
How do I calculate moles?
- Measure the weight of your substance.
- Use a periodic table to find its atomic or molecular mass.
- Divide the weight by the atomic or molecular mass.
- Check your results with Omni Calculator.
How many moles are 3.01 x10 23 molecules of water?
1 mole of any substance has 6.02 x 1023 molecules. For getting the number of moles we can divide 3.01 x 1023/6.02 x 1023 and we receive 0.5 moles.
Why is a mole 6.022 x10 23?
The MOLE (mol) is a unit of measurement that is the amount of a pure substance containing the same number of chemical units (atoms, molecules etc.) as there are atoms in exactly 12 grams of carbon-12 (i.e., 6.022 X 1023).
Converting Between Moles, Atoms, and Molecules
[su_youtube url=”https://www.youtube.com/watch?v=HMAOrGpkTsQ”]
Images related to the topicConverting Between Moles, Atoms, and Molecules
What is the name given to the number 6.022 x10 23?
The number 6.022 × 10²³ is known as Avogadro’s number or Avogadro’s constant. The concept of the mole can be used to convert between mass and number of particles.. Created by Sal Khan.
How many particles are there in 1 mole of water?
A mole (mol) is the amount of a substance that contains 6.02 × 10 23 representative particles of that substance. The mole is the SI unit for amount of a substance. Just like the dozen and the gross, it is a name that stands for a number. There are therefore 6.02 × 10 23 water molecules in a mole of water molecules.
How do you convert molecules to moles?
To go from molecules to moles, divide the numbers of molecules by 6.02 x 1023.
How many molecules of water are there in 180.1 grams of water?
Explanation: The molecular weight of water is 18 (2+16). This means that in 18g of water is one mole. So, in 10 moles, there is 60.23×1023 molecules or 6.023×1024 molecules of water.
How many moles does 80.0 grams of water represent?
how many moles does 80.0 grames of h2o represent? n(H2O)=m(H2O)/Mr(H2O)=80/18=4,44(mol);
How many molecules are in 3 moles of water molecules?
How many water molecules are in 3 moles of water molecules? So, in 3 moles of the substance, there’ll be 3⋅6=18 moles of water molecules. Remember that a mole of molecules contains 6.02⋅1023 molecules. So here, there’ll be a total of 6.02⋅1023⋅18≈1.08⋅1025 water molecules.
How many moles of water are there in 45g of water?
Find the number of moles in 45g of water.
Number of moles of water = Mass of water Gram molecular mass of water =45g18g=2.5.
How many molecules are in 2 moles H2O?
One mole of H₂O contains 6.023 × 10²³ number of H₂O molecules. This is so because 1 mole of any substance contains the amount of 6.023 × 10²³ constituents in it, also known as the Avogadro number. Thus, 2 moles of H₂O will contain 2 × 6.023 × 10²³ number of H₂O molecules, that is 12.046 × 10²³ molecules of H₂O.
How many moles of water are there in 100g of H2O?
There are approximately 5.55 moles of water in a 100-gram sample of water.
How many atoms does 2.0 moles represent?
How many atoms are in 2.00 moles? According to Avogadro’s number, 1 mol of any substance contains 6.022 × 1023 particles. As a result, 2.0 moles of helium (He) represent 2.0 mol × 6.022 × 1023 = 1.2 × 1024 atoms.
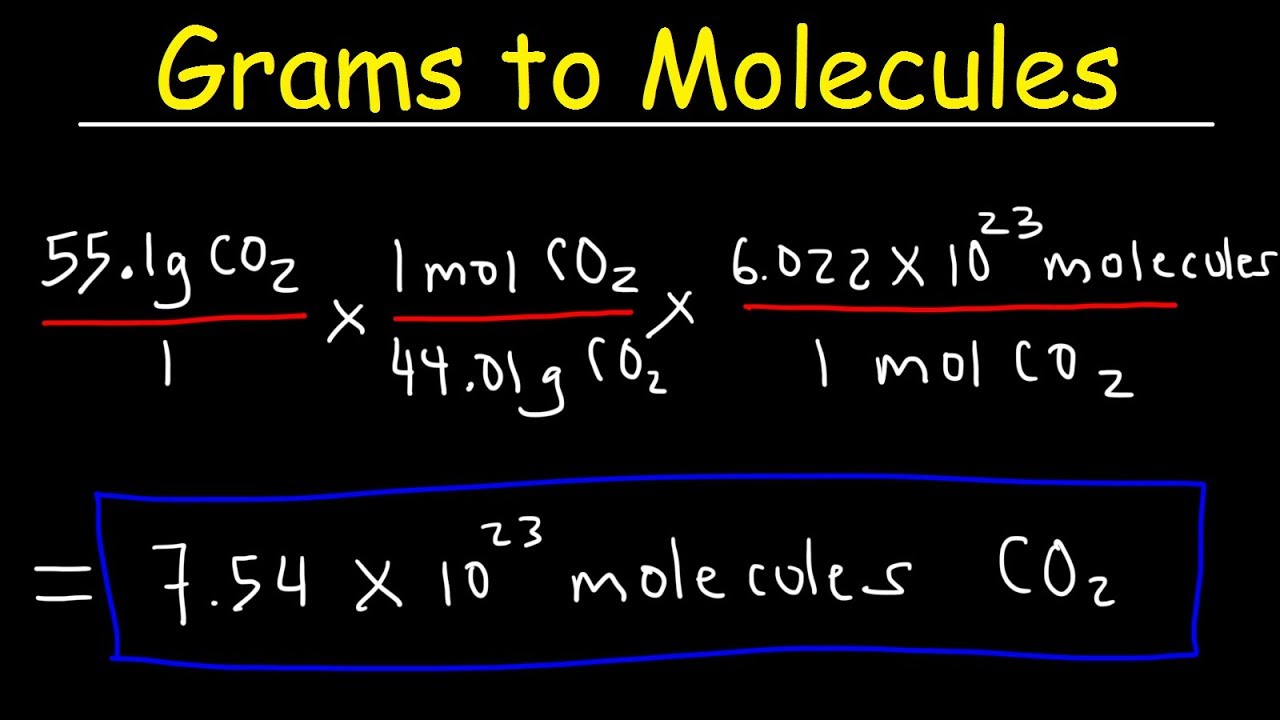
Grams to Molecules and Molecules to Grams Conversion
[su_youtube url=”https://www.youtube.com/watch?v=Ch16s4kYH1Q”]
Images related to the topicGrams to Molecules and Molecules to Grams Conversion
How many helium atoms are in a mole?
A mole of helium gas contains 6.02 x 1023 helium atoms.
How do you convert moles to atoms?
Avogadro’s number is a very important relationship to remember: 1 mole = 6.022×1023 6.022 × 10 23 atoms, molecules, protons, etc. To convert from moles to atoms, multiply the molar amount by Avogadro’s number. To convert from atoms to moles, divide the atom amount by Avogadro’s number (or multiply by its reciprocal).
Related searches
- what is the mass of 1.00 mol of oxygen (o2)
- how many moles water are in 76 1 grams of water
- how many moles water are in 76.1 grams of water?
- what is the mass of 1 00 mol of oxygen o2
- how many moles are in 2.04 x 10^24 molecules of h2o
- molecules to moles
- how many atoms of zinc are in 0.60 mol of zinc
- how many moles are in 150 g of carbon dioxide
- a sample of water has 2 9 1024 molecules in it how many moles of water are in the sample
- convert 2 25 moles of o2 to atoms of o2
- how many atoms of zinc are in 0 60 mol of zinc
- how many atoms are in 4 5 moles of sodium
- molecules to moles calculator
Information related to the topic how many moles is 3.52 x10 24 molecules of water
Here are the search results of the thread how many moles is 3.52 x10 24 molecules of water from Bing. You can read more if you want.
You have just come across an article on the topic how many moles is 3.52 x10 24 molecules of water. If you found this article useful, please share it. Thank you very much.