Let’s discuss the question: how to determine which solution has the lowest freezing point. We summarize all relevant answers in section Q&A of website Abettes-culinary.com in category: MMO. See more related questions in the comments below.

How do you know which element has the lowest freezing point?
That’s due to the fact that Helium has the lowest boiling and freezing points of any other known substance. Helium happens to be the only element that can’t be solidified or frozen at normal atmospheric pressure. Only once you apply a pressure of 25 atmospheres at Helium’s freezing point of −458 °F can you solidify it.
How do you determine the freezing point of a solution?
The freezing point depression ∆T = KF·m where KF is the molal freezing point depression constant and m is the molality of the solute. Rearrangement gives: mol solute = (m) x (kg solvent) where kg of solvent is the mass of the solvent (lauric acid) in the mixture.
Which of these solutions has the lowest freezing point
[su_youtube url=”https://www.youtube.com/watch?v=p8OrLsT6x1g”]
Images related to the topicWhich of these solutions has the lowest freezing point

Which water solution will have the lowest freezing point?
Dividing by 6.02×1023 we can see that the comparative figures are NaCl, 2 , Na2SO4 ,0.9 , glucose , 1.5 , and BaSO4. 1.5 Thus , the NaCl solution has the greatest number of particles per unit volume (considering the ionization of NaCl , Na2SO4andBaSO4) and its will have the lowest freezing point.
Which of the following solutions will have the lowest freezing point?
Therefore, 1.0 M NaCl will cause maximum depression in freezing point and the freezing point of 1.0 M NaCl solution will be lowest.
Why freezing point of solution is less than solvent?
Solutions freezing points are lower than that of the pure solvent or solute because freezing, or becoming solid, creates order and decreases entropy. Solutions have high entropy because of the mix of solvent and solute, so it takes more energy to decrease their entropy to the same point.
Which molal aqueous solution has lowest freezing point?
thus, ΔTf(max) is for 0.10 m alluminium sulphate and Hence, has lowest Freezing point.
How do you determine the freezing point of a molecule?
To compare freezing points, we need to know the total concentration of all particles when the solute has been dissolved. Remember, the greater the concentration of particles, the lower the freezing point will be.
What is KF formula?
Divide the freezing point depression by the molal concentration so you have: Kf = delta Tf / cm. Insert the values for delta Tf and cm. For instance, if you have a solution with a molality of 0.455 which freezes at 3.17 degrees Celsius, then Kf would equal 3.17 divided by 0.455 or 6.96 degrees Celsius.
Which aqueous solution will have the lowest freezing point quizlet?
Which aqueous solution has the lowest freezing point? The solution with the lowest freezing point is 0.25 m AlCl3.
Freezing Point Depression – Chemistry Tutorial
[su_youtube url=”https://www.youtube.com/watch?v=DNy_iD3YqjE”]
Images related to the topicFreezing Point Depression – Chemistry Tutorial
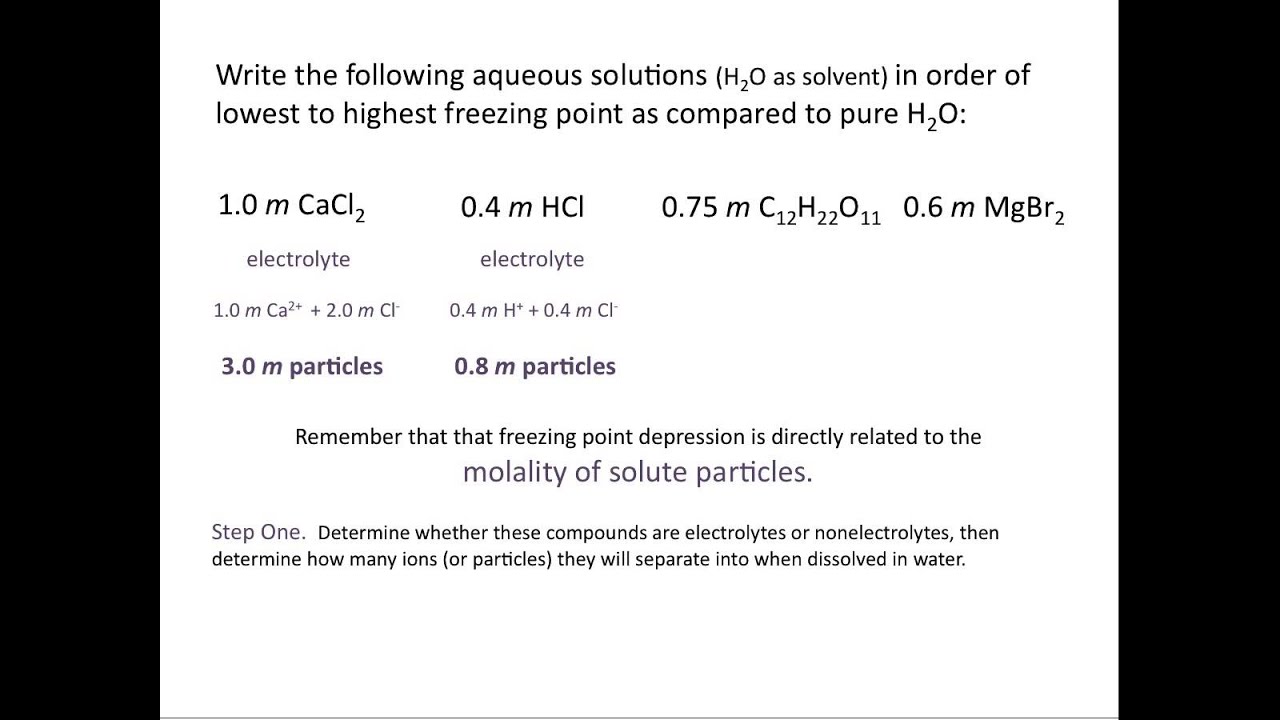
Which solution has the highest freezing point quizlet?
CH3CH2OH is non-electrolyte and doesn’t dissociate into ions and therefore it’s 1.5m concentration is the lowest concentration of dissolved species amongst the answer choices and this solution will have the smallest decrease (depression) in its freezing point leaving it with the overall highest freezing point.
Which of the following would have the lowest freezing point in a solution of 0.5 M?
Of the following 0.5 M, which has the lowest freezing point? dissociates into 4 ions (Al3+ + 3 NO3-). Hence the 0.50 M Al(NO3)3, solution will have the largest concentration of particles in solution and hence the lowest freezing point.
Which of the following has the lowest freezing point assume that all the species are mixed as shown and are completely dissociated?
The correct answer is (b) 1.5 m aluminum nitrate.
What does decreasing freezing point?
Lowering the freezing point allows the street ice to melt at lower temperatures. The maximum depression of the freezing point is about −18 °C (0 °F), so if the ambient temperature is lower, NaCl will be ineffective.
Why is the freezing point of salt water lower than pure water?
Salt water freezes more slowly than pure water because many of the water molecules that would be “crashing” into the surface of the ice in pure water are replaced by these salt ions.
What factor determines how much vapor pressure freezing point and boiling point of a solution differ from those properties of the pure solvent?
To describe the relationship between solute concentration and the physical properties of a solution. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution versus the pure solvent.
Which of the following 0.10 molar aqueous solutions will have the lowest freezing point?
thus, ΔTf(max) is for 0.10 m alluminium sulphate and Hence, has lowest Freezing point.
Colligative Properties – Boiling Point Elevation, Freezing Point Depression \u0026 Osmotic Pressure
[su_youtube url=”https://www.youtube.com/watch?v=c8dDLe37ONg”]
Images related to the topicColligative Properties – Boiling Point Elevation, Freezing Point Depression \u0026 Osmotic Pressure
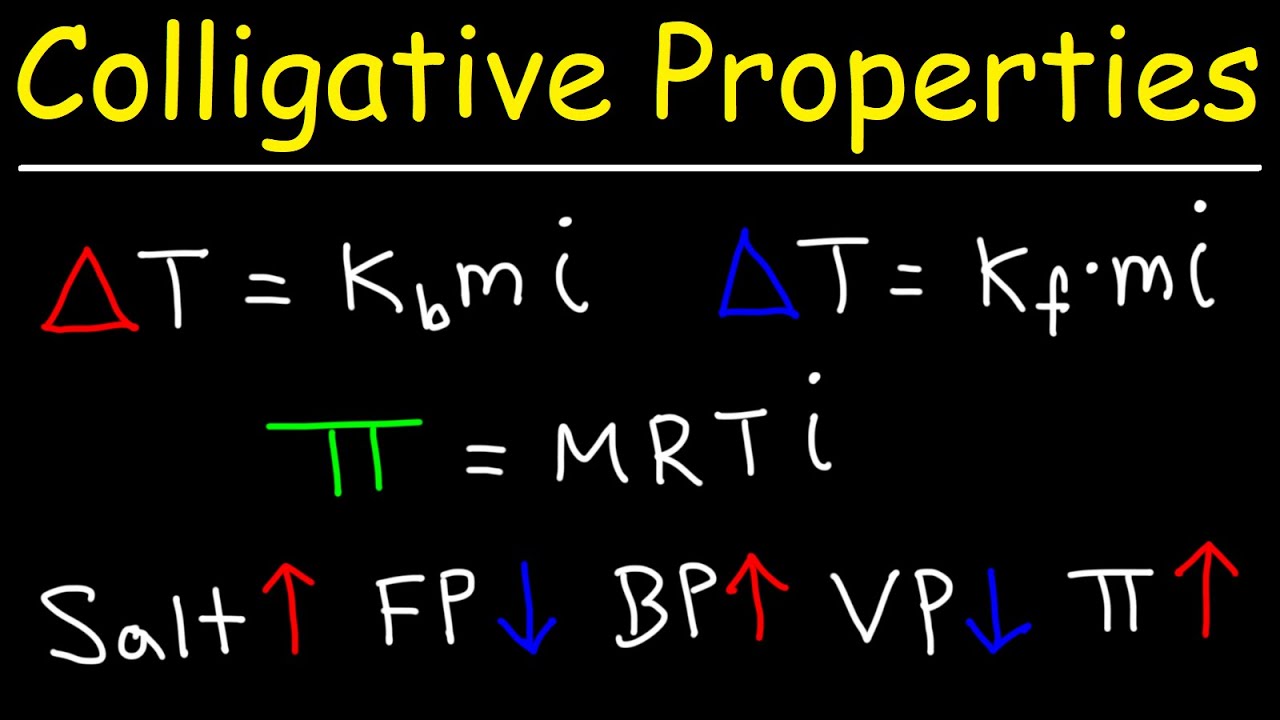
Which aqueous solution has the lowest boiling point?
since C6H12O6 is having the highest value of Vont Hoff factor, $$i$ $; and i is directly proportional to the depression in freezing point hence, C6H12O6 is having the lowest boiling point.
Which has the highest freezing point?
1M glucose solution has the highest freezing point because it has lower ΔTf(ΔTf=Tf∘+ΔTf) since it does not undergo dissociation to increase the number of particles.
Related searches
- which aqueous solution has the lowest freezing point quizlet
- which aqueous solution will have the lowest freezing point?
- which of these solutions has the lowest freezing point
- which of the following has the lowest freezing point quizlet
- how to determine lowest freezing point with molality
- which concentration of a solution of ch3oh in water has the lowest freezing point?
- which of the following aqueous solutions has the lowest freezing point
- which aqueous solution will have the lowest freezing point
- which of these solutions has the highest freezing point
- which of these solutions has the highest freezing point?
- which concentration of a solution of ch3oh in water has the lowest freezing point
Information related to the topic how to determine which solution has the lowest freezing point
Here are the search results of the thread how to determine which solution has the lowest freezing point from Bing. You can read more if you want.
You have just come across an article on the topic how to determine which solution has the lowest freezing point. If you found this article useful, please share it. Thank you very much.