Let’s discuss the question: show that the atomic packing factor for hcp is 0.74.. We summarize all relevant answers in section Q&A of website Abettes-culinary.com in category: MMO. See more related questions in the comments below.
What is the atomic packing factor for HCP?
The majority of metals take on either the hcp, ccp or bcc structure. For fcc and hcp structures, the atomic packing factor is 0.74, which is the maximum packing possible for spheres all having the same diameter.
What is the atomic radius of a HCP crystal structure?
cobalt has an hcp crystal structure, an atomic radius of 0.1253 nm, and a c/a ratio of 1.623.
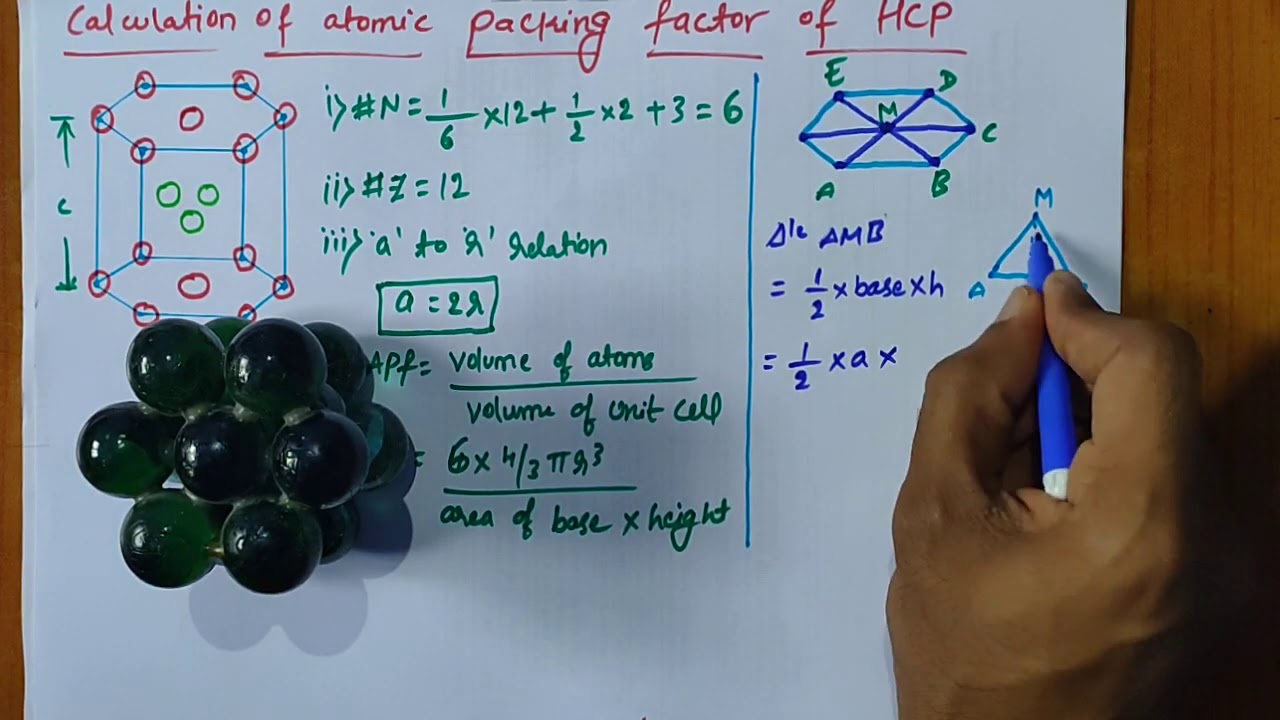
Calculation of atomic packing factor of HCP
[su_youtube url=”https://www.youtube.com/watch?v=tNodTqEZTeg”]
Images related to the topicCalculation of atomic packing factor of HCP

What is the packing efficiency of hcp?
The Packing efficiency of Hexagonal close packing (hcp) and cubic close packing (ccp) is 74%.
What is the coordination number for hcp?
In the hexagonal closest packed (hcp) each ion has 12 neighboring ions hence it has a coordination number of 12 and contains 6 atoms per unit cell.
What is meant by atomic packing factor?
In crystallography, atomic packing factor (APF), packing efficiency, or packing fraction is the fraction of volume in a crystal structure that is occupied by constituent particles. It is a dimensionless quantity and always less than unity.
How many atoms per unit cell are there in an hcp?
The hexagonal closest packed (hcp) has a coordination number of 12 and contains 6 atoms per unit cell.
What is the packing efficiency of HCP in 2d packing?
The packing efficiency of two dimensional hexagon unit cell shown below is se answer: 95.5% 90.7% 76.2% 68.4%
What is a hcp structure?
Hexagonal close packed (hcp) refers to layers of spheres packed so that spheres in alternating layers overlie one another. Hexagonal close packed is a slip system, which is close-packed structure. The hcp structure is very common for elemental metals, including: Beryllium.
Atomic Packing Factor of FCC \u0026 HCP
[su_youtube url=”https://www.youtube.com/watch?v=vOPGHRTWKcY”]
Images related to the topicAtomic Packing Factor of FCC \u0026 HCP

How do you calculate atoms in hcp?
…
- Each Corner atom of hexagonal face is shared by 6 unit cells i.e. they contribute 1/6th of the mass. …
- Thus, contribution of corner atoms = 2 x 6 x 1/6 = 2.
Which of the following has hcp structure?
The metals that are a part of HCP crystal are Zinc, Magnesium, and Cadmium. While metals that are a part of CCP are, Copper, Silver, and Gold, which are coinage metals. Hence, magnesium, Mg is the metal that has HCP structure, so option B is correct.
Which has more packing efficiency hcp or fcc?
The packing efficiency in fcc and hcp structures is 74% . The packing efficiency of a body-centred unit cell is 68% . Packing efficiency of a simple unit cell is 52.4% . Therefore, maximum packing efficiency is shown by hexagonal close packed lattice.
What is CCP and hcp?
The most efficient conformation atomic spheres can take within a unit cell is known as the closest packing configuration. Densely packed atomic spheres exist in two modes: hexagonal closest packing (HCP) and cubic closest packing (CCP).
Is packing efficiency of hcp and fcc same?
Packing fraction of FCC and HCP unit cells are same.
How do you calculate hcp volume?
Volume =63 ×r2× 4r=242 r3.
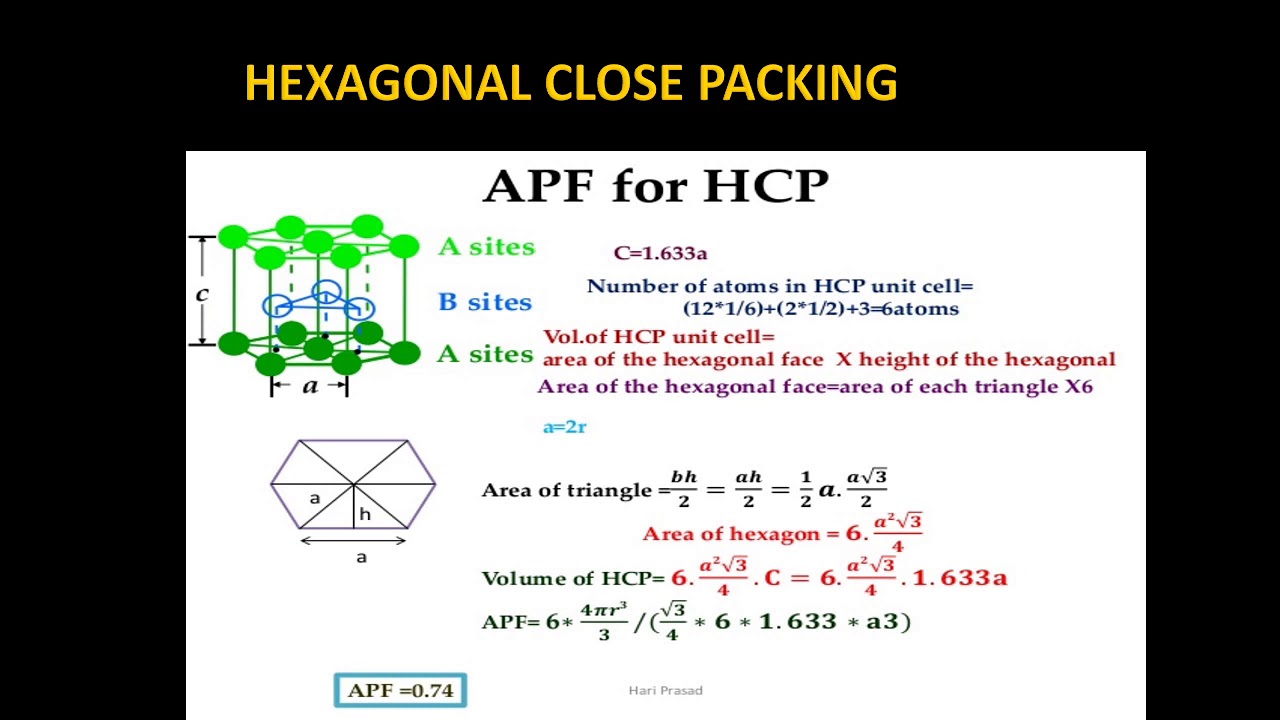
Atomic Packing Factor (HCP)
[su_youtube url=”https://www.youtube.com/watch?v=0Khkv0PCYNs”]
Images related to the topicAtomic Packing Factor (HCP)
What is the full form of hcp in chemistry?
Most metals have structures based on close packed structure types. The three most common are: Hexagonal Close Packed (hcp) (ABAB…layer sequence) (e.g. Zn)
What is the coordination number of hcp and CCP structure?
The co-ordination number of atoms in BCC lattice is 8, in HCP lattice 12, in CCP lattice 12 and in simple lattice it is 6.
Related searches
- show that the atomic packing factor for hcp is 0.74
- show that the atomic packing for hcp is 0.74
Information related to the topic show that the atomic packing factor for hcp is 0.74.
Here are the search results of the thread show that the atomic packing factor for hcp is 0.74. from Bing. You can read more if you want.
You have just come across an article on the topic show that the atomic packing factor for hcp is 0.74.. If you found this article useful, please share it. Thank you very much.